Physicians used point of care rapid immunoassay tests to provide influenza results during H1N1 pandemic
In Apr. 2009, physicians used point of care rapid immunoassay tests to provide influenza results within 15 minutes…

In Apr. 2009, physicians used point of care rapid immunoassay tests to provide influenza results within 15 minutes…
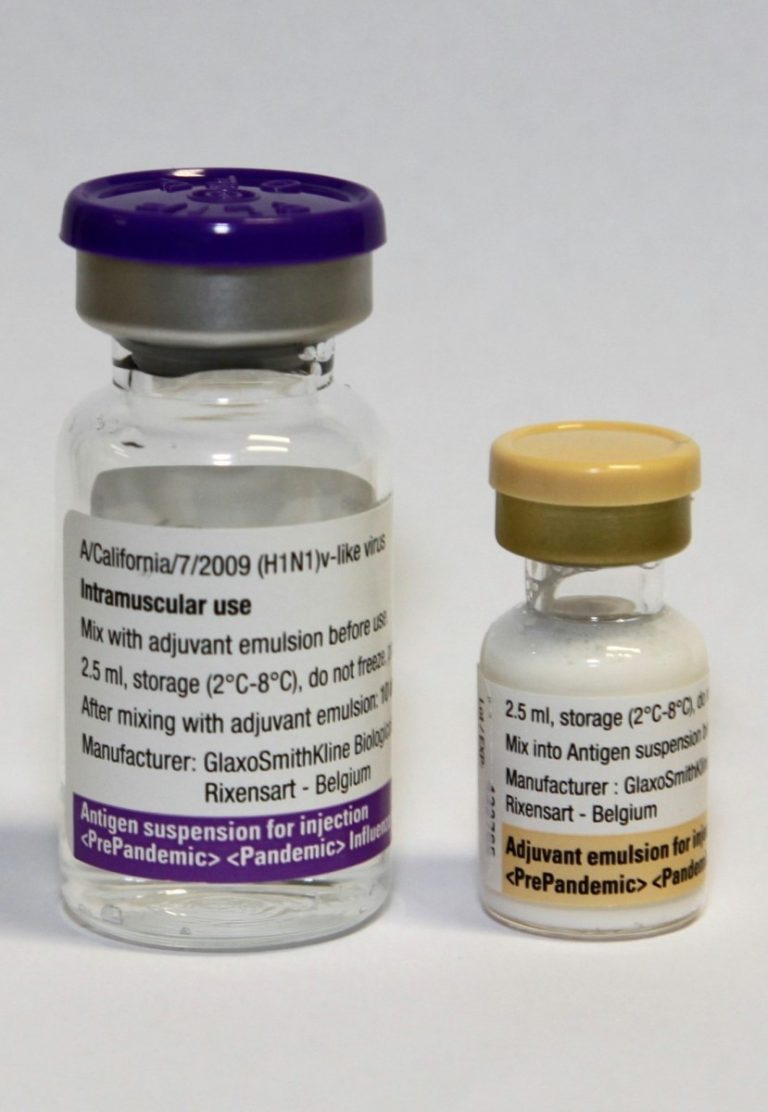
In 2009, an increased risk of narcolepsy (a chronic sleep disorder) was found following vaccination with Pandemrix, a…

On Jun. 23, 2008, Sanofi Pasteur announced the U.S. Food and Drug Administration (FDA) had licensed Pentacel, Diphtheria…

On Mar. 14, 2008, the U.S. Centers for Disease Control and Prevention (CDC) updated its recommendations for administering…
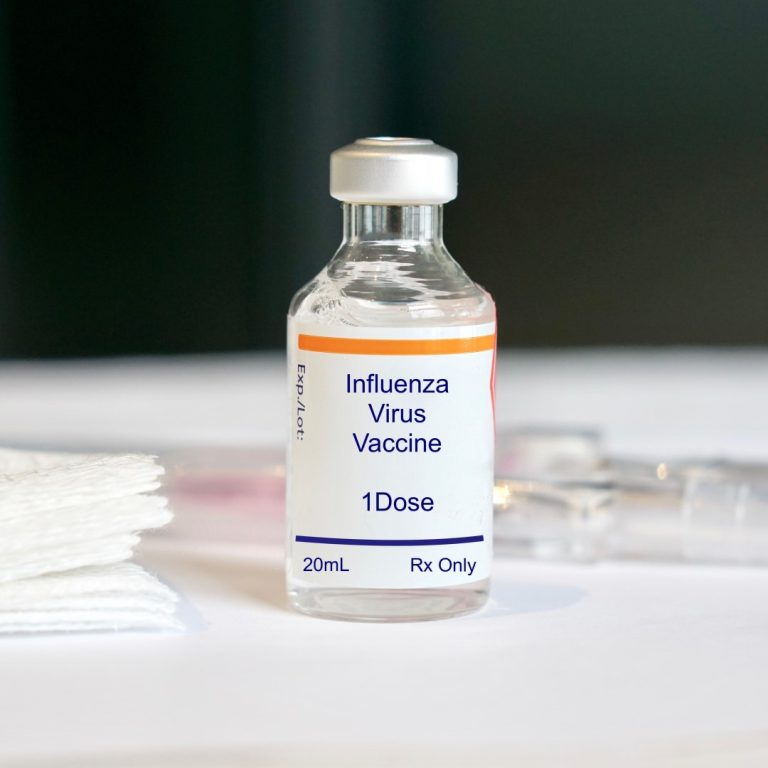
On Feb. 27, 2008, the Immunization Practices Advisory Committee (Immunization Practices Advisory Committee (ACIP) voted to expand influenza…

In 2008, the U.S. Centers for Disease Control and Prevention (CDC) received U.S. Food and Drug Administration (FDA)…

In 2008, the Influenza Reagent Resource (IRR) was established by the Centers for Disease Control and Prevention (CDC)…

On Sept. 20, 2007, the U.S. Food and Drug Administration (FDA) approved use of FluMist nasal-spray influenza vaccine…

On Jun. 7, 2007, years before the COVID-19 pandemic swept across the globe, a group of about 100…

On Feb. 1, 2007, the Centers for Disease Control and Prevention (CDC) released its long-awaited disease containment strategy…

On Jan. 9, 2007, the U.S. Food and Drug Administration (FDA) announced it had approved a refrigerated form…

On Jan. 9, 2007, the Executive Committee of the Council of State and Territorial Epidemiologists approved an interim…
On Jan. 17, 2006, the Centers for Disease Control and Prevention (CDC) announced that it had stopped recommending…
On Oct. 7, 2005, Jeffery Taubenberger, AH Reid, AE Krafft, Karen Bijwaard and Thomas Fanning published a report…

In 2005 and 2006, the White House Homeland Security Council outlined the National Strategy for Pandemic Influenza to…
On Aug. 25, 2004, a significant shortage of influenza vaccine occurred in the U.S. as a result of…

On May 27, 2004, the National Institute of Allergy and Infectious Diseases (NIAID) announced it had awarded contracts…

On Oct. 15, 2003, the Immunization Practices Advisory Committee (ACIP) voted to recommend that children 6 to 23…

On Jun. 17, 2003, the first nasally administered influenza vaccine (FluMist by MedImmune) was licensed. This live influenza…

On Jan. 1, 2003, the Advisory Committee on Immunization Practices (ACIP) encouraged that children 6 to 23 months…
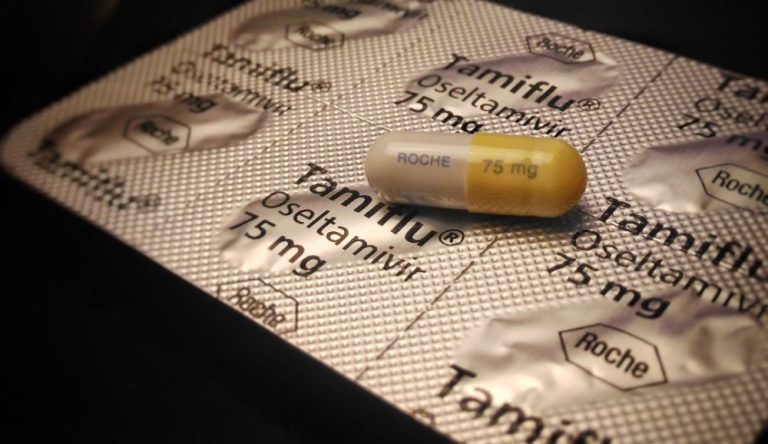
On Oct. 27, 1999, Hoffmann-La Roche and Gilead Sciences announced that Roche’s TAMIFLU (oseltamivir phosphate) had been approved…
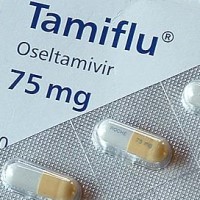
On Oct. 27, 1999, Hoffmann-La Roche and Gilead Sciences announced that Roche’s TAMIFLU™ (oseltamivir phosphate) has been approved…

In April 1999, a pandemic planning framework was published by the World Health Organization (WHO) that emphasized the…

In 1997, FluNet, a global web-based tool for influenza virological surveillance was launched. It is a critical tool…

In 1994, Rimantadine, derived from amantadine, was approved by the Food and Drug Administration (FDA) to treat influenza…
On May 1, 1993, the costs of influenza vaccine and its administration became a covered benefit under Medicare…

On Mar. 1, 1993, the combined Haemophilus influenzae type b vaccine and whole cell DTP vaccine (Tetramune by…
On Dec. 13, 1990, the U.S. Food and Drug Administration (FDA) approved Haemophilus b Conjugate Vaccine (Meningococcal Protein…
On Oct. 4, 1990, the FDA approved the use of Haemophilus b Conjugate Vaccine (Diphtheria CRM 197 Protein…

In 1989, the U.S. Centers for Disease Control and Prevention (CDC) established a World Health Organization (WHO) collaborating…