Moderna filed to expand the conditional Marketing Authorization for COVID-19 vaccine in the EU to include children ages 6-11 years
On Nov. 9, 2021, Moderna announced that it has submitted for a variation to the conditional marketing authorization…

On Nov. 9, 2021, Moderna announced that it has submitted for a variation to the conditional marketing authorization…

On Nov. 9, 2021, Novan announced promising preclinical safety results with berdazimer sodium in a 14-day GLP repeat…

On Nov. 9, 2021, Inovio Pharma announced that the U.S. Food and Drug Administration (FDA) provided authorization to…
On Nov. 8, 2021, Regeneron announced that the European Commission had approved the casirivimab and imdevimab antibody cocktail,…

On Nov. 8, 2021, Amyris announced announced it had entered into a 50:50 joint venture arrangement with ImmunityBio…

On Nov. 8, 2021, The U.S. Food and Drug Administration (FDA) reissued the emergency use authorization (EUA) for…

On Nov. 5, 2021, Pfizer announced it was investigational novel COVID-19 oral antiviral candidate, PAXLOVID, significantly reduced hospitalization…

On Nov. 5, 2021, Chugai Pharmaceutical, announced that it had obtained approval from the Ministry of Health, Labour…

On Nov. 5, 2021, scientists at Oxford University announced they had identified the gene responsible for doubling the…

On Nov. 5, 2021, the U.S. Food and Drug Administration The FDA issued an emergency use authorization (EUA)…

On Nov. 4, 2021, Novavax announced the completion of its rolling submission to the World Health Organization (WHO)…

On Nov. 3, 2021, Innovation Pharma announced that the Company had received confirmation that hard lock of the…

On Nov. 3, 2021, Inovio Pharma announced that it had received authorization from India’s Central Drugs Standard Control…

On Nov. 3, 2021, the World Health Organization (WHO) issued an emergency use listing (EUL) for COVAXINᆴ (developed…
On Nov. 2, 2021, OraSure Technologies announced that the EUA for its InteliSwabル COVID-19 rapid tests had been…
On Nov. 2, 2021, the National Institutes of Health announced support for a four-year follow-up study on the…

On Nov. 1, 2021, for the first time in the U.S., the transmission of COVID-19 from pet parent…

On Nov. 1, 2021, Novavax announced the completion of its rolling submission to Health Canada for authorization of…

On Nov. 1, 2021, Novavax and Serum Institute of India announced that the National Agency of Drug and…

On Nov. 1, 2021, the U.S. Food and Drug Administration (FDA) cleared the first 510(k) for a COVID-19…

On Oct. 31, 2021, Moderna announced that the U.S. Food and Drug Administration (FDA) had notified the Company…
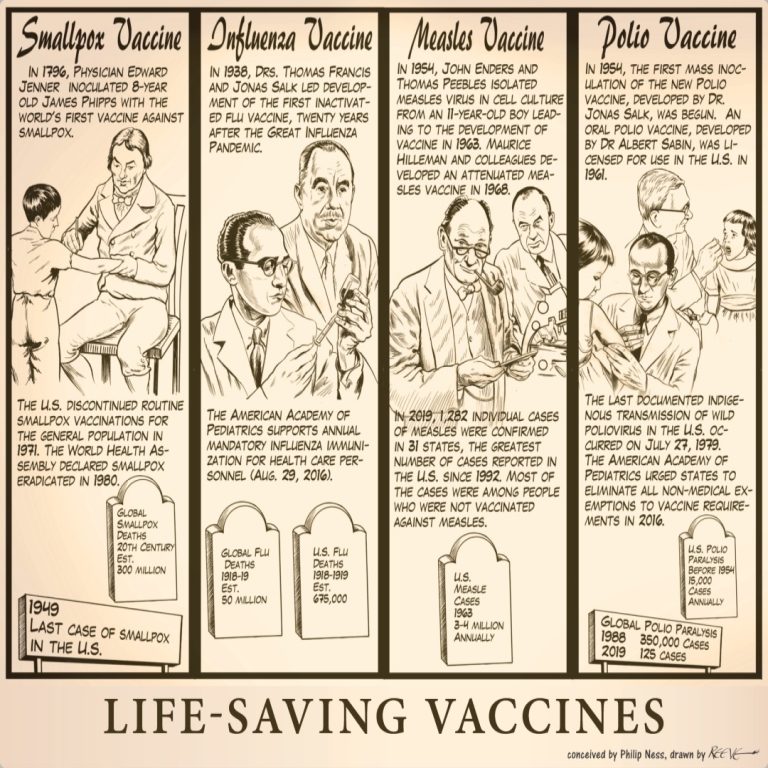
Our Life-Saving Vaccines cartoon illustrates four invaluable vaccines that have affected human civilization throughout history from smallpox and…

On Oct. 29, 2021, Oregon Health & Science University’s (OHSU) board of directors approved a project to expand…

On Oct. 29, 2021, Novavax announced the completion of its rolling submission to the Therapeutic Goods Administration (TGA)…
On Oct. 29, 2021, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) had authorized…
On Oct. 29, 2021, the Centers for Disease Control and Prevention (CDC) published “new science” reinforcing that vaccination…

On Oct. 28, 2021, Pfizer and BioNTech announced that the U.S. government had purchased 50 million more doses…

On Oct. 27, 2021, researchers published interim results in The New England Journal of Medicine from a Phase…

On Oct. 27, 2021, the Medicines Patent Pool (MPP) and Merck announced a voluntary licensing agreement to facilitate…
On Oct. 27, 2021, Hologic announced that its Aptima SARS-CoV-2/Flu Assay was available for the simultaneous detection and…