FDA issued Emergency Use Authorization to Yale School of Public Health for SalivaDirect method of saliva sample processing
On Aug. 15, 2020, the FDA issued an emergency use authorization (EUA) to Yale School of Public Health…

On Aug. 15, 2020, the FDA issued an emergency use authorization (EUA) to Yale School of Public Health…
On Aug. 14, 2020, AstraZeneca concluded an agreement with the European Commission (EC) to supply up to 400…

On Aug. 14, 2020, Medigen Vaccine Biologics (MBV) published preclinical results of their COVID-19 vaccine candidate in BioRxiv,…
On Aug. 14, 2020, Novavax announced it had signed a Heads of Terms (Term Sheet) with the Government…

On Aug. 13, 2020, data from the WHO/UNICEF Joint Monitoring Programme (JMP) revealed that 43 per cent of…

On Aug. 13, 2020, Royal Philips introduced its Rapid Equipment Deployment Kit for ICU ramp-ups, allowing doctors, nurses,…

On Aug. 13, 2020, Dynavax Technologies announced a grant from the Bill & Melinda Gates Foundation of $3.4…
On Aug. 13, 2020, Novavax and SK bioscience announced a development and supply agreement for the antigen component…

On Aug. 13, 2020, Heat Biologics reported preclinical data for Heat’s gp96-based COVID-19. The data, generated at the…

On Aug. 13, 2020, the U.S. Department of Health and Human Services (HHS) announced combined investments of $6.5…

On Aug. 12, 2020, Baxter announced it had received Emergency Use Authorizations (EUAs) from the FDA for the…

On Aug. 12, 2020, Thermo Fisher Scientific announced it had committed to supporting the efforts of historically black…

On Aug. 11, 2020, Hologic announced that it has validated use of its Aptimaᆴ and Panther Fusionᆴ molecular…

On Aug. 11, 2020, LabCorpᆴ announced details of a no charge antibody testing program in response to federal…
On Aug. 10, 2020, Gilead Sciences announced that it had submitted a New Drug Application (NDA) to the…

On Aug. 10, 2020, Vaxart announced that its COVID-19 Investigational New Drug (IND) application has been filed with…

On Aug. 10, 2020, CBRᆴ by Generate Life Sciences, the worldメs largest private newborn stem cell bank, and…

On Aug. 10, 2020, Humanigen announced that the Brazilian regulatory agency, Anvisa, had granted permission to commence a…

On Aug. 10, 2020, Omeros reported the results of a compassionate-use study evaluating narsoplimab, Omerosメ investigational human monoclonal…

On Aug. 9, 2020, OpGen announced that it had commenced marketing and promotion, on a non-exclusive basis, of…
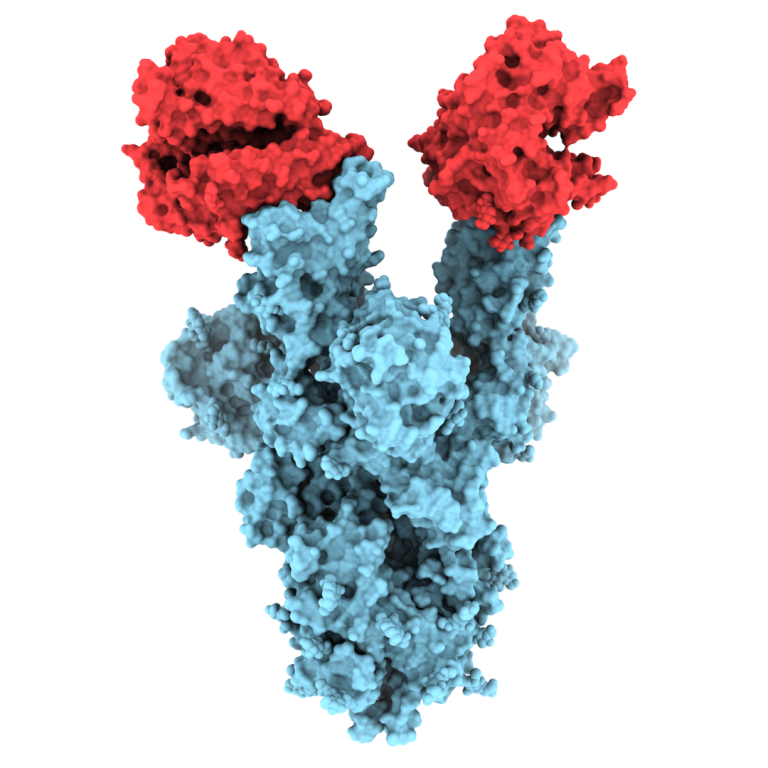
On Aug. 7, 2020, scientists at Scripps Research announced they had obtained high-resolution, atomic-scale details of the structure…
On Aug. 7, 2020, Novavax and Takeda Pharmaceutical announced a partnership for the development, manufacturing and commercialization of…

On Aug. 7, 2020, Pfizer announced a multi-year agreement with Gilead Sciences to manufacture and supply Gileadメs investigational…

On Aug. 6, 2020, RedHill Biopharma announced approval from the Mexican Federal Committee for the Protection against Sanitary…

On Aug. 6, 2020, researchers at the AIDS Institute, Department of Microbiology and State Key Laboratory of Emerging…

On Aug. 6, 2020, Novavax announced it had entered a supply and license agreement with the Serum Institute…

On Aug. 6, 2020, Organicell Regenerative Medicine announced that the FDA had approved two outpatient Emergency Investigational New…

On Aug. 5, 2020, a randomized, controlled clinical trial evaluating the safety and efficacy of a treatment regimen…

On Aug. 5, 2020, the National Institutes of Health (NIH) announced it had launched the Medical Imaging and…

On Aug. 5, 2020, the National Institute of Allergy and Infectious Diseases (NIAID) announced that the investigational vaccine…