World Health Organization approved SK bioscience as supplier of Novavax Nuvaxovid COVID-19 vaccine
On Sept. 2, 2022, Novavax announced that the World Health Organization (WHO) had approved a variation to allow…

On Sept. 2, 2022, Novavax announced that the World Health Organization (WHO) had approved a variation to allow…

On Sept. 1, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had been recommended for expanded conditional marketing…

On Aug. 31, 2022, the Yale New Haven Hospital broke ground on the $838 million, 505,000 square foot…

On Aug. 26, 2022, Novavax announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the United…

On Aug. 26, 2022, Pfizer and BioNTech announced they had completed a submission to the European Medicines Agency…

On Aug. 23, 2022, Moderna announced that it has completed its submission to the U.S. Food and Drug…

On Aug. 23, 2022, Pfizer and BioNTech announced updated efficacy results from a Phase 2/3 trial evaluating a…

On Aug. 23, 2022, Moderna announced that the Government of Canada had exercised its option to purchase an…
On Aug. 22, 2022, Pfizer and BioNTech announced they had completed a submission to the U.S. Food and…
On Aug. 19, 2022, Novavax announced that the Novavax COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) had received expanded emergency use…

On Aug. 18, 2022, Novavax announced that New Zealand’s Medsafe had granted expanded provisional approval for Nuvaxovid (NVX-CoV2373)…

On Aug. 15, 2022, Moderna announced that the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK…

On Aug. 12, 2022, Novavax announced that partner, SK bioscience, had received a Post Approval Change Application approval…

On Aug. 9, 2022, Moderna announced an amendment to its agreement with the European Commission (EC) to convert…

On Aug. 4, 2022, Novavax announced the initiation of its Phase 2b/3 Hummingbird global clinical trial. The trial…

On Jul. 29, 2022, the U.S. Department of Health and Human Services (HHS), in collaboration with the U.S….

On Jul. 27, 2022, Pfizer and BioNTech announced that the companies had initiated a randomized, active-controlled, observer-blind, Phase…

On Jul. 26, 2022, Novavax announced that the Australian Therapeutic Goods Agency had granted expanded approval for provisional…

On Jul. 26, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had received expanded manufacturing and marketing approval…
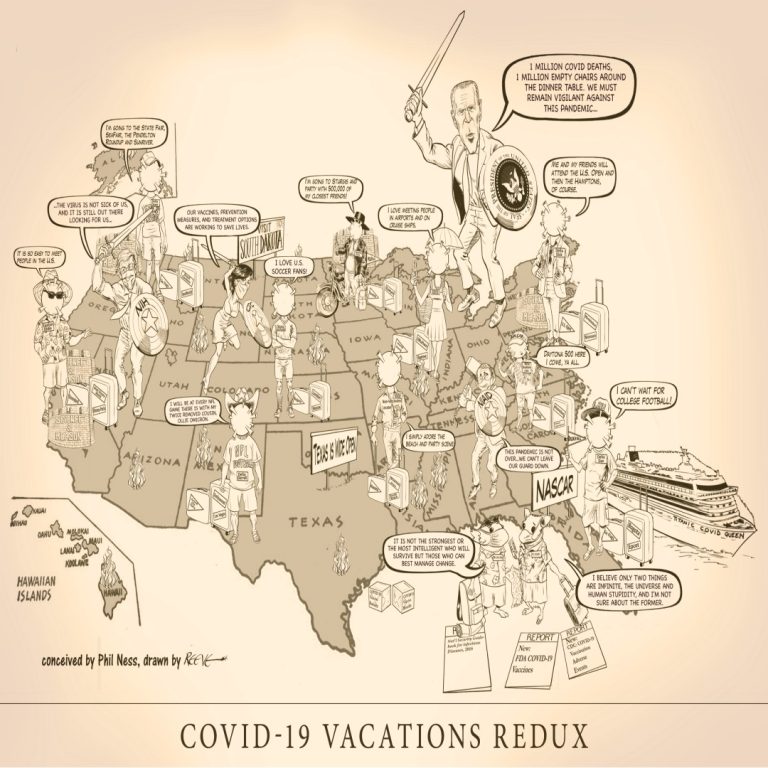
COVID-19 Vacations Redux iIlustrates once again the pits and perils vacation travelers face as they begin their Summer…
On Jul. 22, 2022, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…
On Jul. 22, 2022, Gilead Sciences announced that the Committee for Medicinal Products for Human Use (CHMP) of…
On Jul. 19, 2022, Novavax announced that it had signed agreements with its partner, SK bioscience, for the…

On Jul. 19, 2022, Novavax announced that the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee…
On Jul. 19, 2022, Gilead Sciences and the European Commission announced a new joint procurement agreement (JPA) that…
On Jul. 19, 2022, the NIH announced that although COVID-19 booster vaccinations in adults elicit high levels of…
On Jul. 18, 2022, Moderna announced that the Therapeutic Goods Administration (TGA) in Australia had granted provisional registration…

On Jul. 14, 2022, Moderna announced that Health Canada had approved the use of Moderna’s mRNA COVID-19 vaccine,…

On Jul. 13, 2022, Novavax announced that the European Commission (EC) had approved the expanded conditional marketing authorization…

On Jul. 13, 2022, Novavax announced that its COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) had received emergency use authorization from…