FDA Approved First Gene Therapy for Treatment of Aromatic L-amino Acid Decarboxylase Deficiency
On Nov. 14, 2024, the U.S. Food and Drug Administration approved PTC Therapeutics’ Kebilidi (eladocagene exuparvovec-tneq), an adeno-associated…

On Nov. 14, 2024, the U.S. Food and Drug Administration approved PTC Therapeutics’ Kebilidi (eladocagene exuparvovec-tneq), an adeno-associated…
On Oct. 11, 2024, Pfizer announced that the U.S. Food and Drug Administration (FDA) had approved HYMPAVZI™ (marstacimab-hncq)…
On Aug. 6, 2024, the U.S. Food and Drug Administration (FDA) announced it had approved vorasidenib (Voranigo, Servier…

On Apr. 3, 2024, the U.S. Food and Drug Administration approved Basilea Pharmaceutica’s Zevtera (ceftobiprole medocaril sodium for…

On Dec. 18, 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced a study that showed…

On Nov. 16. 2023, the U.S. Centers for Disease Control and Prevention (CDC) announced that Following years of…
On Nov. 9, 2023, the U.S. Food and Drug Administration approved ‘ Takeda PharmaceuticalsVAdzynma, the first recombinant (genetically…
On Oct. 20, 2023, the U.S. Centers for Disease Control and Prevention (CDC) reported that among children and…

On Oct. 14, 2023, Purdue University ensured that the memory of the former graduate and devoted Boilermaker football…

On Jun. 22, 2023, the U.S. Food and Drug Administration (FDA) approved Sarepta Therapeutics’s Elevidys, the first gene…

On Jun. 20, 2023, the U.S. Food and Drug Administration (FDA) approved Boehringer Ingelheim’s Jardiance (empagliflozin) and Synjardy…

On Apr. 17, 2023, the U.S. FDA approved Gamida Cell’s Omisirge (omidubicel-onlv), a substantially modified allogeneic (donor) cord…
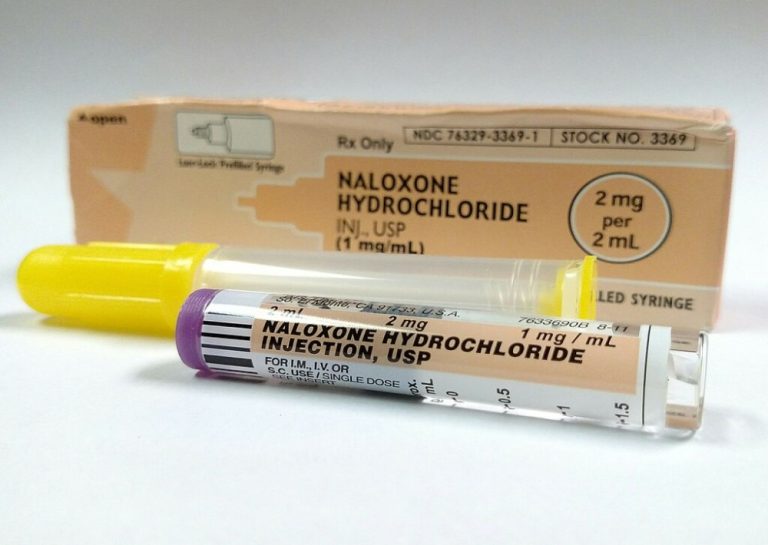
On Mar. 8, 2023, Amphastar Pharmaceuticals announced that the U.S. Food and Drug Administration (FDA) had granted approval…

On Mar. 1, 2023, BioNTech and Pfizer announced that they had submitted an application to the U.S. Food…

On Dec. 16, 2022, Moderna announced that the European Medicines Agency’s Committee for Medicinal Products for Human Use…
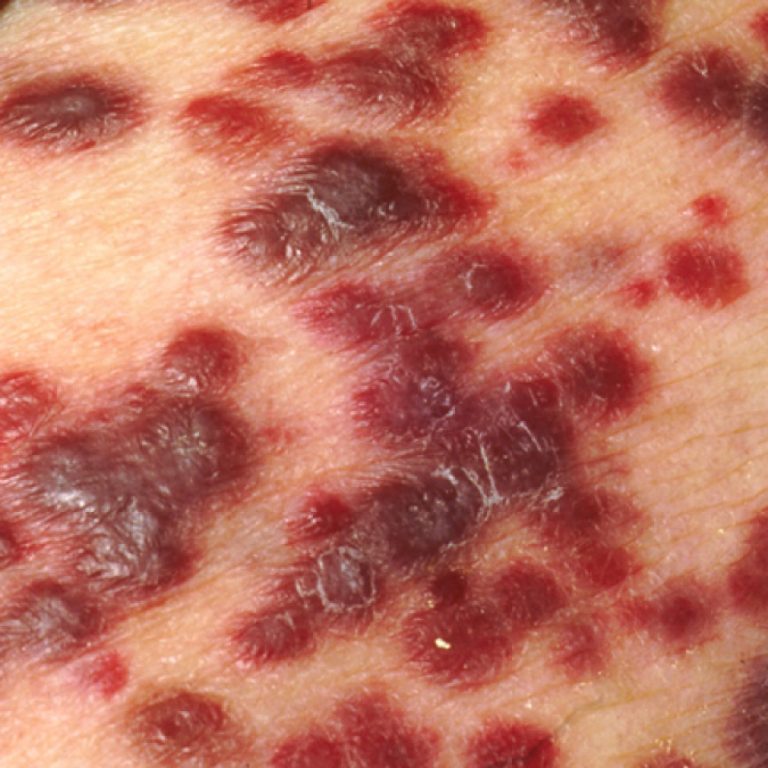
On Dec. 9, 2022, the U.S. Food and Drug Administration (FDA) approved Genentech’s atezolizumab (Tecentriq) for adult and…

On Nov. 17, 2022, the U.S. Food and Drug Administration (FDA) approved Tzield (teplizumab-mzwv) injection to delay the…

On Aug. 17, 2022, the U.S. Food and Drug Administration approved Zynteglo (betibeglogene autotemcel) developed by bluebird bio,…

On Jun. 23 2022, Novavax announced the filing of a Supplement to a New Drug Submission with Health…
On Apr. 25, 2022, Gilead Sciences announced that the U.S. Food and Drug Administration (FDA) had approved a…

On Apr. 1, 2022, the Fred Hutchinson Cancer Research Center and Seattle Cancer Care Alliance (SCCA) announced a…

On Mar. 31, 2022, Novavax announced submission of its request to expand the conditional marketing authorization (CMA) of…

On Mar. 23, 2022, Moderna announced positive interim data from the Phase 2/3 KidCOVE study of the Moderna…

On Mar. 21, 2022, in support of the Cancer Moonshot? goal of fostering data sharing in cancer research,…

On Mar. 21, 2022, in support of the Cancer Moonshot goal of fostering data sharing in cancer research,…
On Mar. 9, 2022, Pfizer announced that it had initiated a Phase 2/3 study, EPIC-PEDS (Evaluation of ProteaseInhibition…

On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…
On Feb. 11, 2022, Gilead Sciences announced new data from an interim analysis of its ongoing, Phase 2/3…

On Feb. 10, 2022, Novavax announced that NVX-CoV2373, its recombinant nanoparticle protein-based COVID-19 vaccine, achieved its primary effectiveness…
On Jan. 20, 2022, Gilead Sciences announced that the U.S. Food and Drug Administration had granted expedited approval…