Moderna announced contract with Brazil to supply 12.5 million COVID-19 vaccines as part of national vaccination campaign
On Apr. 22, 2024, Moderna announced a contract with the Ministry of Health in Brazil (Ministerio da Saude)…
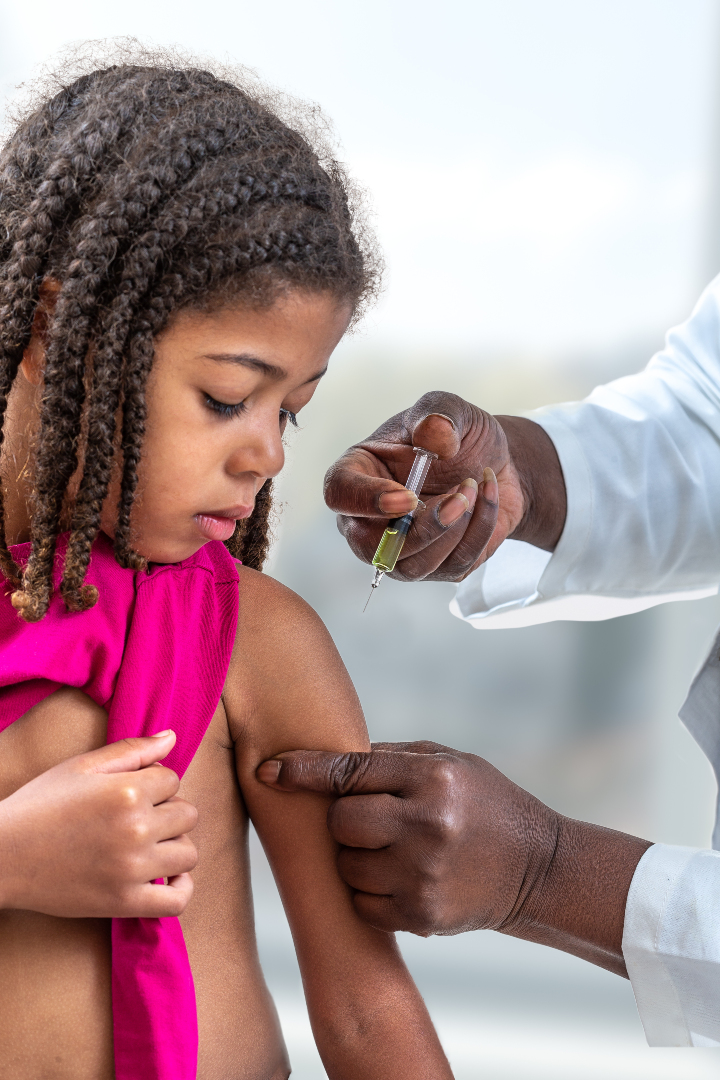
On Apr. 22, 2024, Moderna announced a contract with the Ministry of Health in Brazil (Ministerio da Saude)…
On Apr. 15, 2024, scientists at University of California Riverside demonstrated a new, RNA-based vaccine strategy that is…
On Feb. 28, 2024, the U.S. Centers for Disease Control and Preventionメs (CDC) Advisory Committee on Immunization Practices…
On Feb. 14, 2024, the National Institute of Allergy and Infectious Diseases (NIAID) reported study results that showed…
On Feb. 5, 2024, CSL and and Arcturus Therapeutics announced the results of a follow-up analysis of a…
On Jan. 31, 2024, researchers at the National Institutes of Health detected abnormal proteins in the spinal fluid…

On Jan. 10, 2024, an National Institutes of Health (NIH) supported study announced an n atlas revealing the…

On Dec. 21, 2023, CSL and and Arcturus Therapeutics announced the results of a Phase 3 study showing…

On Dec. 18, 2023, COVID-19 vaccine maker BioNTech (22UAy.DE) announced that it aims to start production at its…

On Dec. 14, 2023, the University of Alberta announced that a study published in Nature sheds light on…

On Dec. 7, 2023, BioNTech announced that it had signed a multi-year strategic partnership with the State of…
On Nov. 28, 2023, CSL and Arcturus Therapeutics announced that Japan’s Ministry of Health, Labor and Welfare (MHLW)…

On Oct. 18, 2023, Novavax announced that Singapore’s Health Sciences Authority (HSA) had granted full approval for Novavax’s…

On Oct. 3, 2023, the U.S. Food and Drug Administration (FDA) amended the emergency use authorization (EUA) of…

On Oct. 2, 2023, the Nobel Prize in Physiology or medicine was awarded jointly to Katalin Kariko and…

On Sept. 28, 2023, Pfizer and BioNTech that Health Canada had authorized the companies’ Omicron XBB.1.5-adapted monovalent COVID-19…

On Sept. 27, 2023, Gritstone bio announced that it was awarded a contract by the Biomedical Advanced Research…

On Sept. 11, 2023, Pfizer and BioNTech announced that the U.S. Food and Drug Administration (FDA) approved the…

On Aug. 30, 2023, Pfizer and BioNTech announced that the Committee for Medicinal Products for Human Use (CHMP)…
On Aug. 11, 2023, a study funded by the National Institute of Allergy and Infectious Diseases (NIAID) reported…

On Jul. 25, 2023, U.S. National Science Foundation-supported research, led by Joshua Rosenthal of the Marine Biological Laboratory…

On Jul. 18, 2023, scientists reported that they had extracted, sequenced, and analyzed historical RNA from muscle and…
On Apr. 27, 2023, a NIAID-led Phase 2 trial compared two Pfizer bivalent mRNA COVID-19 boosters found that…
On Apr. 25, 2023, the U.S. FDA approved Qalsody (tofersen) to treat patients with amyotrophic lateral sclerosis (ALS)…

On Mar. 30, 2023, Moderna and the Government of the Republic of Kenya announced they had finalized an…
On Mar. 15, 2023, scientists at Texas A&M announced that a research study: ‘RNA interference is essential to…

On Feb. 17, 2023, Moderna announced that Health Canada had authorized the use of its Omicron-targeting bivalent COVID-19…

On Feb. 9, 2023, the National Institutes of Health (NIH) announced that a new large-scale genetic analysis found…
On Jan. 17, 2023, Moderna announced positive topline data from its ConquerRSV Phase 3 pivotal efficacy trial of…
On Jan. 5, 2023, BioNTech announced that the Company had signed a Memorandum of Understanding with the Government…