Abbott received CE Mark for its COVID-19 IgG quantitative antibody blood test
On Jan. 11, 2021, Abbott announced it had received FDA 510(k) clearance for the first rapid handheld traumatic…

On Jan. 11, 2021, Abbott announced it had received FDA 510(k) clearance for the first rapid handheld traumatic…

On Nov. 10, 2020, 3M announced that its TB Quat Disinfectant Ready-to-Use Cleaner had been approved by the…

On Oct. 22, 2020, the World Health Organizationメs Global TB Programme welcomed the results from a landmark study…
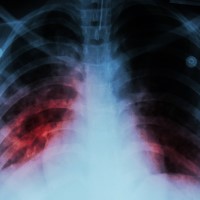
On Oct. 21, 2020, results from an international, randomized, controlled clinical trial indicated that a four-month daily treatment…
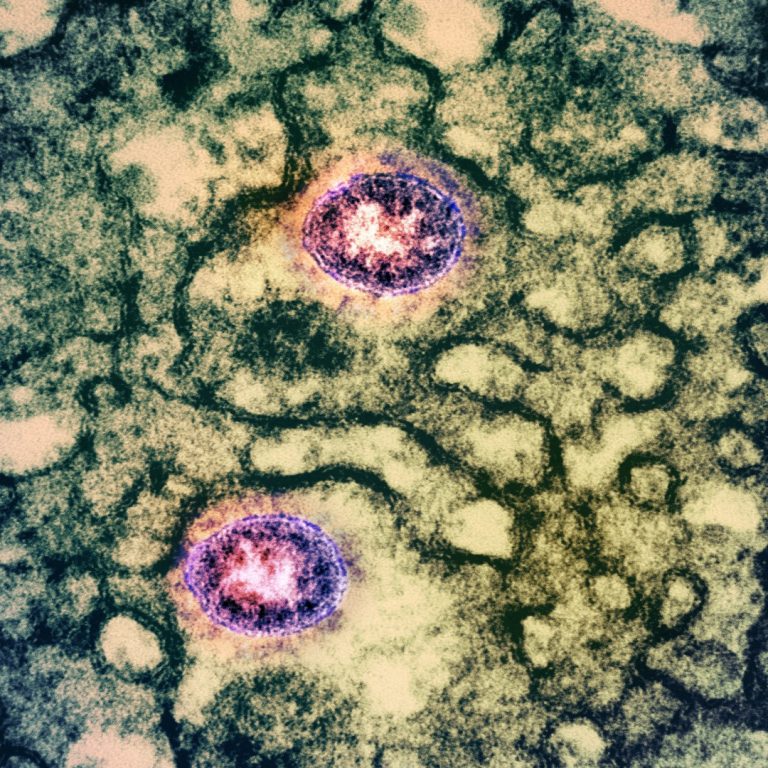
On Oct. 16, 2020, BD (Becton, Dickinson) announced the CE mark and European availability of a product for…
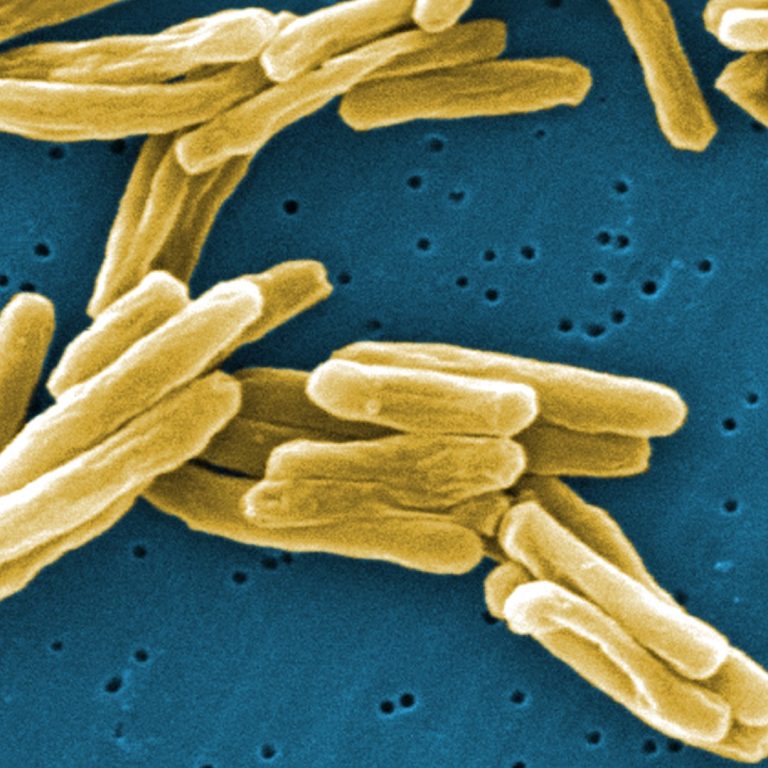
On Oct. 14, 2020, the World Health Organization (WHO) announced that prior to the COVID-19 pandemic, many countries…

On Sept. 29, 2020, Oxford Immunotec announced that it had received clearance from the FDA to amend the…

On Jul. 20, 2020, Oxford Immunotec announced that it had entered into a long-term agreement to supply its…

On Jul. 9, 2020, a study from the NIH showed that neurofilament light chain (NfL) delivered superior diagnostic…

On Jul. 9, 2020, the CDC reported that after a decade of increasing rates, contact sportsヨrelated TBI ED…
On May 27, 2020, the National Institutes of Health (NIH) announced it had found that molecules released into…

On May 1, 2020, XBiotech announced that human immune donors had been identified that can support the Company’s…
On Apr. 29, 2020, a team of Texas A&M University researchers asked hundreds of frontline medical workers to…
On Apr. 21, 2020, University of Oxford announced that it had successfully obtained re-registration of the T-SPOT.TB test…
On Mar. 20, 2020, the Centers for Disease Control and Prevention (CDC) reported that tuberculosis (TB) incidence in…

On Feb. 27, 2020, a consortium of philanthropic, non-profit and private sector organisations launched a collaboration that aims…

On Jan. 27, 2020, GSK announced that it had licensed its M72/AS01E[3] tuberculosis disease (TB) vaccine candidate to…

On Jan. 17, 2020, the Non-profit drug developer TB Alliance granted Shenyang Hongqi Pharmaceuticals , a subsidiary of…

On Oct. 24, 2019, Penn Medicine researchers received $9.7 million grant from the NIH to study traumatic brain…

On Sept. 26, 2019, Seattle Children’s announced it was one of three recipients of $30 million in first-year-funding…
On May 7, 2019, Chembio Diagnostics announced an agreement with Perseus Science Group, to advance the development of…
On Jan. 7, 2019, the Emory University School of Medicine received a $500,000 grant from the Centers for…
On Nov. 1, 2004, an Oregon Health & Science University (OHSU) research team announced that it was one…

On Apr. 15, 1999, the Tennessee Biotechnology Association (TBA), now the Life Science Tennessee, was established. The Tennessee…

In March 1998, the Stop TB Initiative was established following a meeting of the First Ad hoc Committee…

On Apr. 14, 1994, the Alpha-Tocopherol Beta-Carotene (ATBC) Cancer Prevention Study found no benefit from the use of…

In 1991, a sharp increase of tuberculosis was reported by the U.S. Centers for Disease Control and Prevention…

In 1990, the U.S. Centers for Disease Control and Prevention (CDC) reported a large outbreak of of nosocomial…
In December 1988, the National Cancer Institute (NCI) sponsored a workshop to address the standardization of cervical/vaginal cytopathology…

In May 1978, the U.S. Centers for Disease Control and Prevention (CDC) reported a patient was first diagnosed…