Anixa Biosciences and OntoChem entered next stage of development for Covid-19 therapeutic
On Apr. 5, 2021, Anixa Biosciences announced that based on Proof of Concept animal study results, it was…

On Apr. 5, 2021, Anixa Biosciences announced that based on Proof of Concept animal study results, it was…
On Apr. 5, 2021, Novavax announced the initiation of crossover arms in two ongoing clinical trials of NVX-CoV2373,…
On Apr. 1, 2021, Arbutus Biopharma, X-Chem and Proteros Biostructures announced hat they had entered into a discovery…
On Apr. 1, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that an investigational vaccine designed to…

On Mar. 30, 2021, CureVac and Celonic Group announced their partnership for the production of CureVacメs mRNA-based COVID-19…
On Apr. 1, 2021, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that an investigational vaccine designed to…

On Mar. 30, 2021, Qx Therapeutics announced that the FDA had cleared the companyメs Investigational New Drug application…

On Mar. 29, 2021, an international research team led by Oxford University scientists announced they had developed a…
On Mar. 25, 2021, an international team of scientists announced they had found evidence that SARS-CoV-2 infects cells…
On Mar. 25, 2021, about two decades after first devising a new kind of vaccine, Oregon Health &…

On Mar. 24, 2021, Virginia Tech researchers announced that their candidate Coronavirus vaccines, prevented pigs from becoming ill…

On Mar. 23, 2021, Pfizer announced that it was progressing to multiple ascending doses after completing the dosing…

On Mar. 22, 2021, CureVac announced plans to expand and further specify the protocols of its ongoing late-stage…

On Mar. 22, 2021, Oxford University announced that a Phase III study of the Oxford-AstraZeneca coronavirus vaccine conducted…

On Mar. 21, 2021, Cepheid announced it had received Emergency Use Authorization from the U.S. Food & Drug…

On Mar. 19, 2021, the FDA issued an emergency use authorization to Tiger Tech Solutions for the first…

On Mar. 17, 2021, Tonix Pharma announced preliminary results following vaccination of non-human primates with TNX-1800 (modified horsepox…

On Mar. 17, 2021, Altasciences working with the National Research Council of Canada (NRC), announced that it had…

On Mar. 15, 2021, ImmunityBio announced it had met the safety requirements for the first 12 participants in…

On Mar. 11, 2021, ImmunityBio announced it was developing a novel hAd5 ACE2 Decoy therapeutic vaccine to neutralize…

On Mar. 10, 2021, CEPI, the Coalition for Epidemic Preparedness Innovations, and VBI Vaccines announced a partnership to…
On Mar. 10, 2021, Innovation Pharmaceuticals announced that a Machine Learning (Artificial Intelligence) model used to screen 1,482…
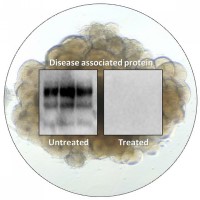
On Mar. 9, 2021, the NIH announced that approximately two years after establishing a human cerebral organoid system…

On Mar. 9, 2021, NanoViricides announced that NV-CoV-2 and NV-CoV-2-R were found to be highly effective against a…

On Mar. 9, 2021, VBI Vaccines announced the initiation of enrollment of its Phase 1/2 clinical study of…

On Mar. 8, 2021, ImmunityBio and NantKwest announced that the first cohorts of their South Africa and U.S….

On Mar. 5, 2021, Abbott announced the U.S. Food and Drug Administration’s (FDA) Emergency Use Authorization (EUA) for…

On Mar. 5, 2021, Merck and Ridgeback Biotherapeutics, announced preliminary results from Ridgebackメs Phase 2a randomized, double-blind, placebo-controlled…

On Mar. 5, 2021, the U.S. Food and Drug Administration issued an emergency use authorization (EUA) for the…

On Mar. 5, 2021, Innovation Pharmaceuticals reported that eight sites were participating in the Company’s international Phase 2…