Preclinical study showed SARS-CoV-2 spike protein licensed by Oragenics from NIH produces neutralizing antibodies
On Jul. 22, 2020, Oragenics announced that the National Institutes of Health created stabilized pre-fusion spike protein (CoV-2…

On Jul. 22, 2020, Oragenics announced that the National Institutes of Health created stabilized pre-fusion spike protein (CoV-2…

On Jul. 21, 2020, National Institutes of Health (NIH) researchers reported early results from the first COVID-19 vaccine…
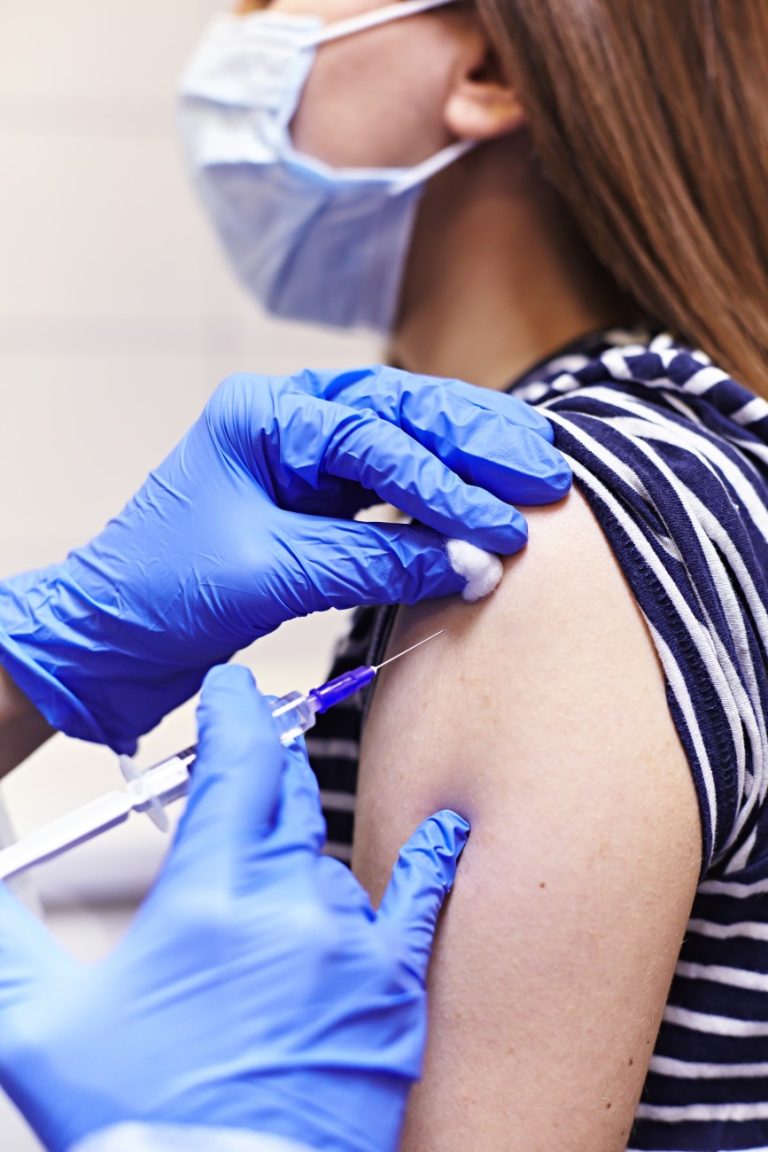
On Jul. 21, 2020, researchers from the National Institutes of Health (NIH) announced that early results from the…

On Jul. 21, 2020, Bio-Techne announced that Exosome Diagnostics, a Bio-Techne brand, had completed validation testing for COVID-19…

On Jul. 20, 2020, AstraZeneca announced that Interim results from the ongoing Phase I/II COV001 trial, led by…

On Jul. 20, 2020, interim results from the ongoing Phase I/II COV001 trial, led by Oxford University, showed…

On Jul. 20, 2020, Luminex announced the FDA had issued an Emergency Use Authorization for the company’s xMAPᆴ…

On Jul. 20, 2020, scientists at HDT Bio, PAI Life Sciences, and the University of Washington announced they…

On Jul. 16, 2020, Heron Therapeutics announced initiation of the GUARDS-1 Study, a Phase 2 clinical study evaluating…

On Jul. 16, 2020, Codex DNA announced the release of a new synthetic SARS-CoV-2 genome developed to accelerate…

On Jul. 16, 2020, Tonix Pharmaceuticals announced it had entered into a research collaboration and option agreement with…
On Jul. 15, 2020, bioMerieux announced that the BIOFIRE Respiratory Panel 2.1 plus (RP2.1plus) was CE marked. The…
On Jul. 15, 2020, scientists at Washington University School of Medicine in St. Louis reported they had joined…
On Jul. 15, 2020, AXIM Biotechnologies announced the development of NeuCovixTM, a rapid diagnostic test measuring levels of…

On Jul. 14, 2020, a study by NIH researchers reported the placental membranes that contain the fetus and…
On Jul. 14, 2020, an investigational vaccine, mRNA-1273, designed to protect against SARS-CoV-2, the virus that causes coronavirus…

On Jul. 14, 2020, in an editorial published in the Journal of the American Medical Association (JAMA), the…

On Jul. 14, 2020, Dynavax Technologies announced that the first participants have been dosed in the Phase 1…

On Jul. 14, 2020, 3M and researchers at MIT announced they had started testing a new rapid test…
On Jul. 14, 2020, Pascal Biosciences announced it had discovered certain cannabinoids that block replication of SARS-CoV-2, the…

On Jul. 13, 2020, a team of researchers from Oxford University, the Rosalind Franklin Institute, Diamond Light Source…

On Jul. 13, 2020, an emerging virus threatens both wild and pet rabbits in the U.S. The fatal…

On Jul. 13, 2020, Altimmune announced positive results from the preclinical studies of its intranasal COVID-19 vaccine candidate,…

On Jul. 13, 2020, Pfizer and BioNTech announced that two of the companiesメ four investigational vaccine candidates from…

On Jul. 9, 2020, Atos announced that the Biocomputing Unit at the Spanish National Center for Biotechnology (CNB),…

On Jul. 9, 2020, BioSig Technologies and its subsidiary, ViralClear Pharma announced that it is partnering with Albany…

On Jul. 8, 2020, Kaiser Permanente Washington Health Research Institute (KPWHRI) announced that it had created a registry…

On Jul. 8, 2020, Kleo Pharmaceuticals announced that it had received a $5 million grant from the Bill…

On Jul. 8, 2020, NanoViricides announced that excellent safety and tolerability of the drug candidates it is developing…
On Jul. 7, 2020, scientists from the University of Oxford’s Department of Engineering Science and Oxford Suzhou Centre…