Abbott launched molecular point-of-care test to detect novel Coronavirus in as Little as five minutes
On Mar. 27, 2020, Abbott announced the FDA has issued Emergency Use Authorization (EUA) for the fastest available…

On Mar. 27, 2020, Abbott announced the FDA has issued Emergency Use Authorization (EUA) for the fastest available…

On Mar. 27, 2020, University of Oxford researchers working in an unprecedented vaccine development effort to prevent COVID-19…

On Mar. 27, 2020, Abbott announced that the FDA had issued Emergency Use Authorization (EUA) for the fastest…
On Mar. 27, 2020, in response to the crisis caused by the COVID-19 virus in California and around…

On Mar. 27, 2020, Humanigen announced that the company had submitted an initial protocol synopsis to U.S. Food…
On Mar. 27, 2020, Luminex announced the U.S. Food and Drug Administration (FDA) issued an Emergency Use Authorization…

On Mar. 26, 2020, Thermo Fisher Scientific announced it had received the CE mark in the European Union…

On Mar. 26, 2020, Dynavax and the Coalition for Epidemic Preparedness Innovations (CEPI) announced a collaboration supporting the…
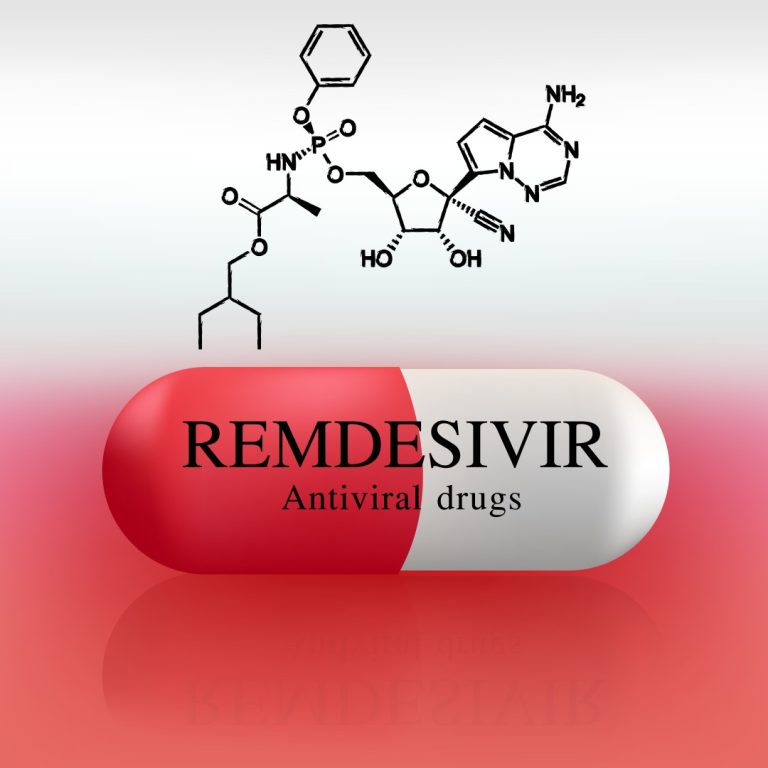
On Mar. 26, 2020, physician-scientists at four University of California Health medical centers — UC San Diego Health,…
On Mar. 25, 2020, the National Library of Medicine (NLM), part of the National Institutes of Health, announced…

On Mar. 25, 2020, employees of Sartorius Stedim Biotech in Aubagne, France are keeping production running to ensure…

On Mar. 25, 2020, Philips announced that it was increasing the production of certain critical care products and…

On Mar. 25, 2020, a mobility and epidemiological study from a global consortium of researchers, led by the…
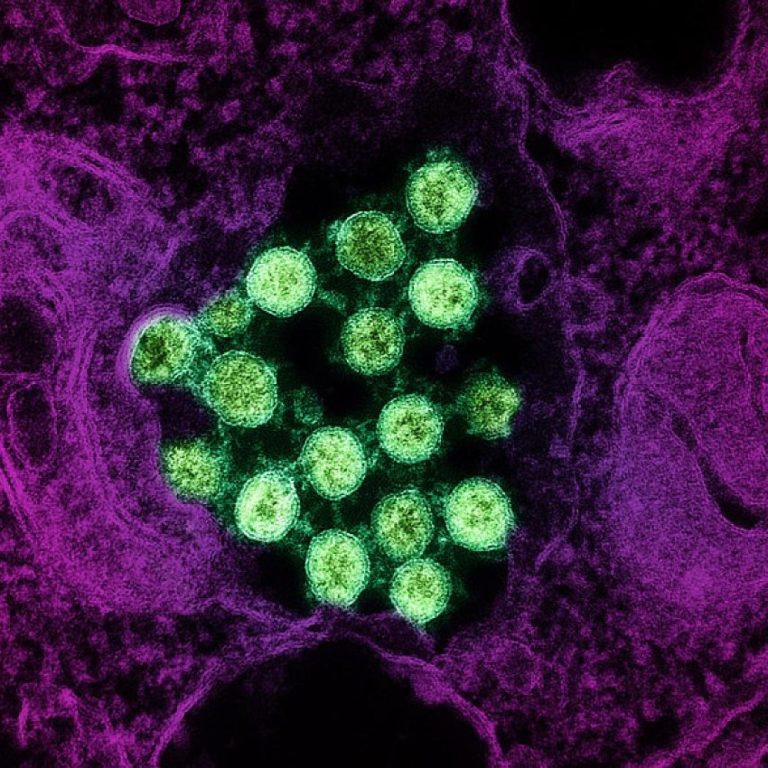
On Mar. 25, 2020, Mateon Therapeutics announced an update on its rapid antiviral response program targeting coronaviruses, initially…

On Mar. 25, 2020, Vir Biotech announced announced that it has identified multiple human monoclonal antibody (mAb) development…

On Mar. 25, 2020, Xencor announced a technology license agreement with Vir Biotech in which Vir will have…

On Mar. 25, 2020, CHF Solutions announced the use of its Aquadex therapy in the treatment of patients…

On Mar. 25, 2020, Merck announced it had donated 300,000 masks to New Jersey’s Office of Homeland Security…

On Mar. 25, 2020, the Perelman School of Medicine at the University of Pennsylvania announced it had established…

On Mar. 25, 2020, Mylan announced that it had waived its exclusive rights in the U.S. to distribute…

On Mar. 24, 2020, T2 Biosystems announced it has entered into a worldwide licensing agreement for a rapid…

On Mar. 24, 2020, T2 Biosystems announced it had entered into a worldwide licensing agreement for a rapid…
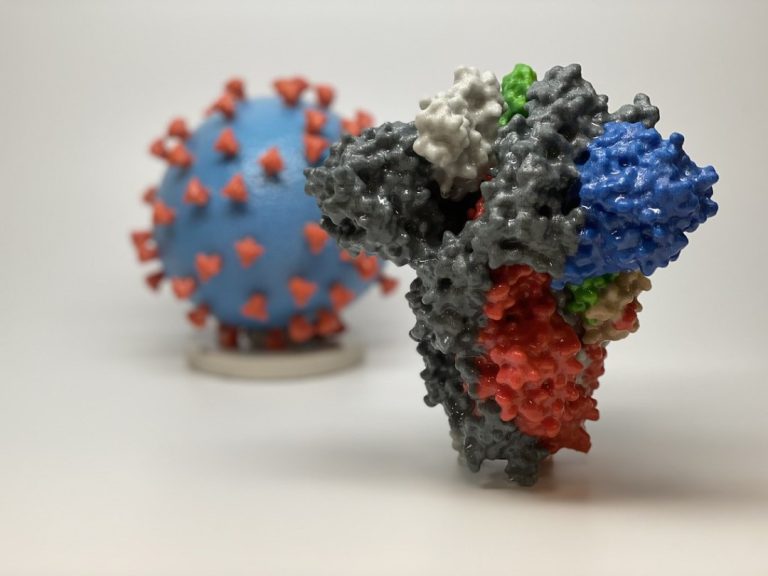
On Mar. 24, 2020, to help in educating the public and in the hope of better conveying the…
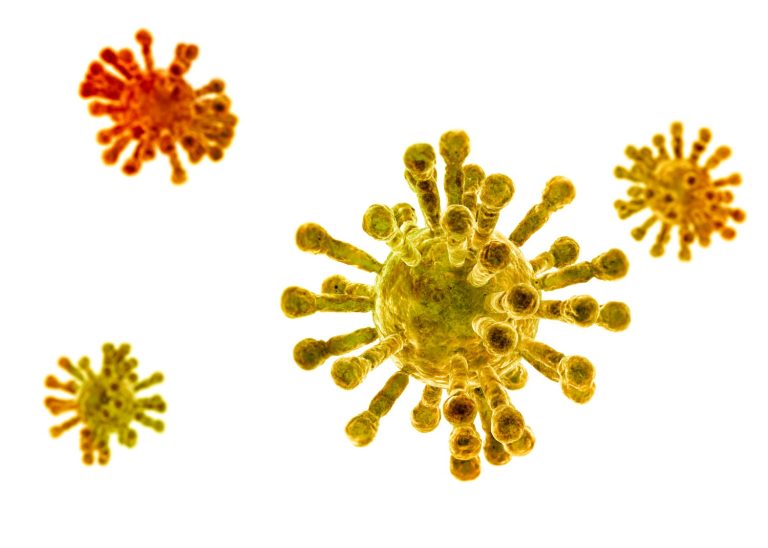
On Mar. 24, 2020, City of Hope announced that it had employed its vast expertise using the immune…

On Mar. 24, 2020, PerkinElmer announced the U.S. Food and Drug Administration (FDA) had provided Emergency Use Authorization…

On Mar. 24, 2020, a new international project aims to enroll 500 COVID-19 patients to search for genetic…

On Mar. 24, 2020, three Oxford University-based COVID-19 projects were among the first to benefit from a share…

On Mar. 24, 2020, Dynavax Technologies and Clover Biopharmaceuticals, a China-based global clinical-stage biotechnology company, announceda research collaboration…

On Mar. 24, 2020, Mesa Biotech announced it had received Emergency Use Authorization (EUA) from the FDA for…

On Mar. 24, 2020, BioSig Technologies announced that its majority-owned subsidiary NeuroClear Technologies, acquired the rights to develop…