Monkeys developed protective antibodies to SARS-CoV-2
On Mar. 17, 2020, a small study of macaques found they don’t develop a coronavirus infection the second…

On Mar. 17, 2020, a small study of macaques found they don’t develop a coronavirus infection the second…
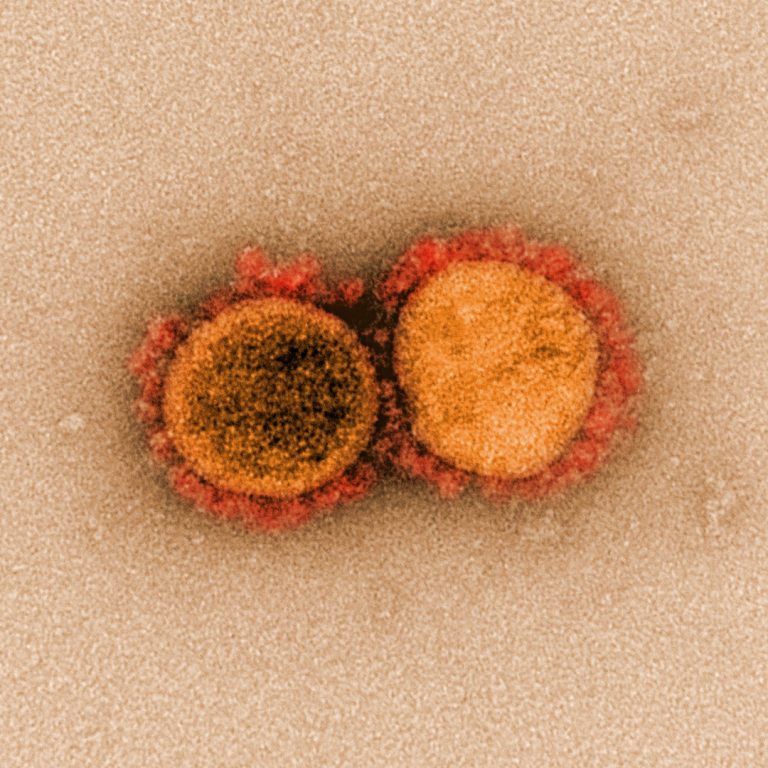
On Mar. 17, 2020, CanSino Biologics announced that its recombinant novel Coronavirus Vaccine (Adenovirus Type 5 Vector) candidate…
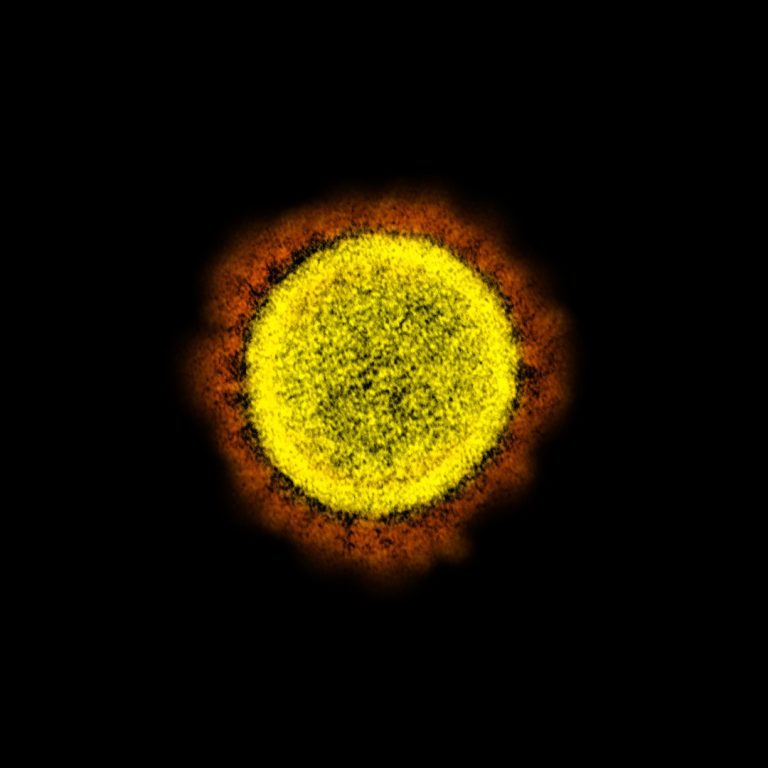
On Mar. 17, 2020, scientists reported the novel SARS-CoV-2 coronavirus that emerged in the city of Wuhan, China,…
On Mar. 17, 2020, Innovation Pharma announced details on the Material Transfer Agreement signed with a leading public…

On Mar. 17, 2020, BioMedomics announced it will be able to distribute their COVID-19 IgM-IgG Antibody Rapid Test…

On Mar. 17, 2020, BioReference Laboratories, an OPKO Health company, announced a collaboration with the New York City…

On Mar. 17, 2020, Pfizer and BioNTech SE announced the companies have agreed to a letter of intent…

On Mar. 17, 2020, Regeneron announced that its scientists had isolated hundreds of virus-neutralizing, fully human antibodies from…
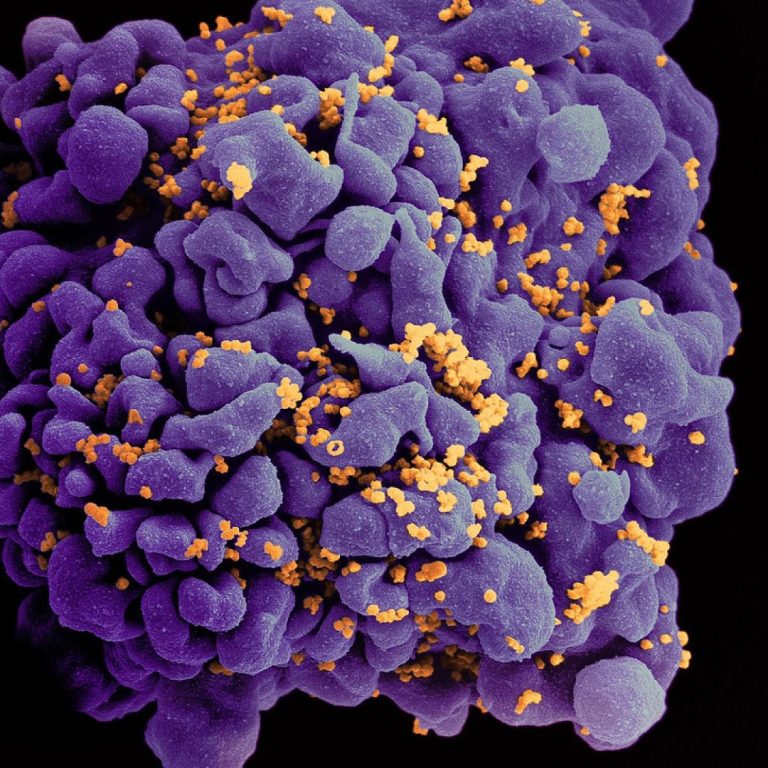
On Mar. 16, 2020, the Hormel Institute, University of Minnesota announced published research concerning remnants of ancient viruses…
On Mar. 16, 2020, the National Institutes of Health (NIH announced that a Phase 1 clinical trial evaluating…

On Mar. 16, 2020, Moderna announced that the first participant had been dosed in the Phase 1 study…

On Mar. 16, 2020, Hologic announced the U.S. Food and Drug Administration (FDA) had granted Emergency Use Authorization…

On Mar. 16, 2020, BD (Becton, Dickinson and Company) announced the companies had submitted Emergency Use Authorization requests…

On Mar. 16, 2020, PathGroup announced it had begun to offer testing for Coronavirus Disease 2019 (COVID-19). This…
On Mar. 15, 2020, the National Institutes of Health (NIH) informed its staff that it had its first…

On Mar. 15, 2020, CureVac announced that internal efforts will be focused on the development of a coronavirus…

On Mar. 14, 2020, CureVac announced positive pre-clinical results at a low dose for its lead vaccine candidate…

On Mar. 13, 2020, Roche announced the FDA has issued an Emergency Use Authorization for the cobasᆴ SARS-CoV-2…

On Mar. 13, 2020, Thermo Fisher Scientific announced the FDA has issued an emergency use authorization (EUA) for…

On Mar. 13, 2020, the Federal Government, along with State and local governments, announced preventive and proactive measures…
On Mar. 13, 2020, Iceland health authorities and deCode Genetics announced they began comprehensive screening for the virus…
On Mar. 13, 2020, Enanta Pharmaceuticals announced it had initiated a program to discover direct-acting antiviral drug candidates…

On Mar. 13, 2020, IDEXX Laboratories announced the company had seen no positive results in pets to date…
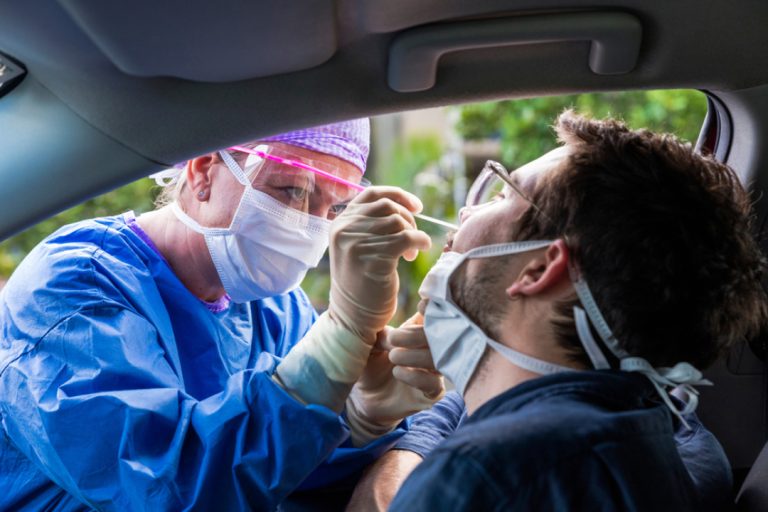
On Mar. 13, 2020, BioReference Laboratories, an OPKO company, announced it had begun accepting specimens for testing of…

On Mar. 13, 2020, Luminex announced that four independent clinical laboratories had validated laboratory developed tests (LDTs) for…
On May 12, 2022, the National IHR Focal Point for Qatar reported two laboratory-confirmed cases of Middle East…

On Mar. 12, 2020, Pluristem Therapeutics announced it a collaborative agreement with the BIH Center for Regenerative Therapy…
On Mar. 12, 2020, Vir Biotech announced it had signed a letter of intent with Biogen for the…

On Mar. 12, 2020, AbCellera and Eli Lilly announced an agreement to co-develop antibody products for the treatment…

On Mar. 12, 2020, Innovation Pharma announced research procedures that one of the 12 Regional Biocontainment Labs (RBLs)…