AIM ImmunoTech received Orphan Drug Designation for Ampligen (rintatolimod) for treatment of Ebola virus disease
On Nov. 2, 2022, AIM ImmunoTech announced that the U.S. Food and Drug Administration had granted Orphan Drug…

On Nov. 2, 2022, AIM ImmunoTech announced that the U.S. Food and Drug Administration had granted Orphan Drug…
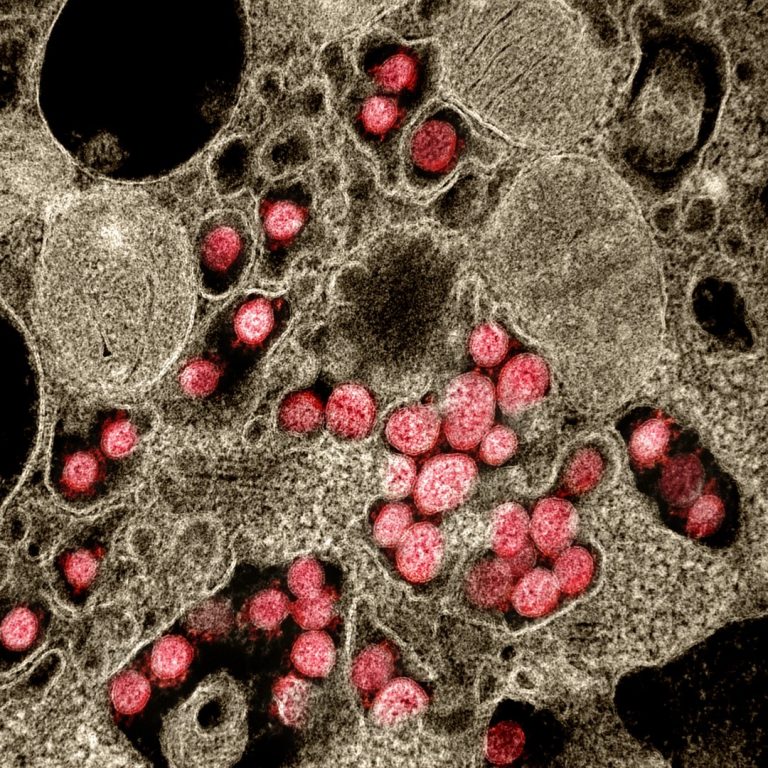
On Oct. 25, 2022, Pacific Northwest National Laboratory (PNNL) announced that scientists have shown that they can detect…
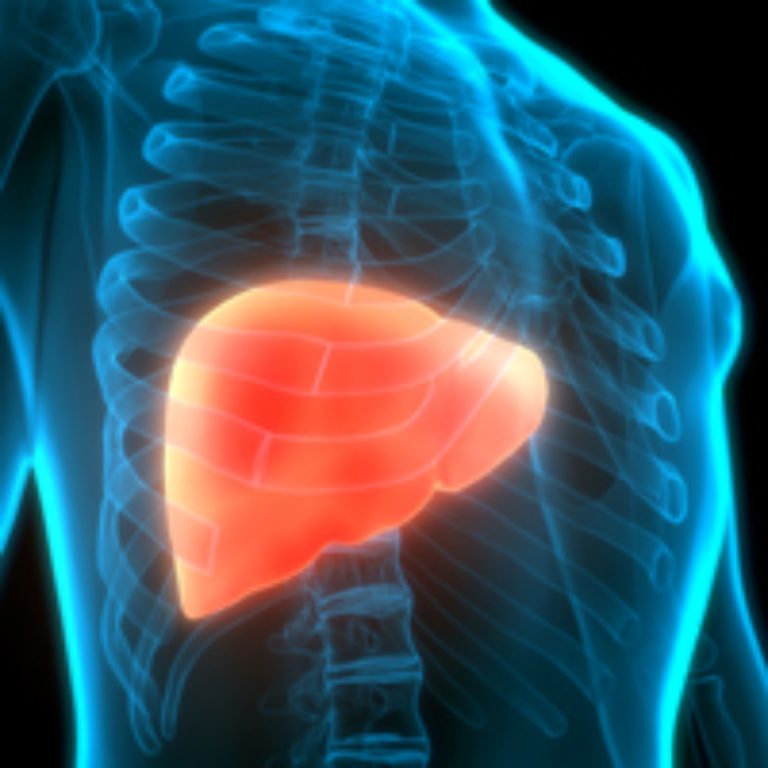
On Oct. 20, 2022, the National Institutes of Health announced that a three-dose course of the hepatitis B…

On Oct. 19, 2022, Moderna announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human…
On Oct. 12, 2022, Moderna announced that it had received emergency use authorization from the U.S. Food and…

On Oct. 11, 2022, researchers from the University of Oxford have reported new findings from a Phase 1…

On Oct. 10, 2022, Novavax announced that partner, SK bioscience, had submitted a Post Approval Change Application to…
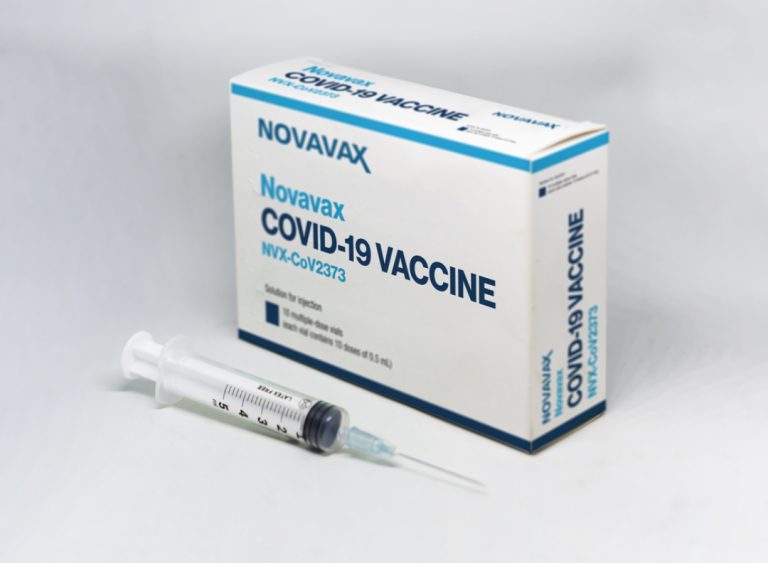
On Oct. 10, 2022, Novavax announced that Switzerland’s Federal Office of Public Health had recommended Nuvaxovid (NVX-CoV2373) as…
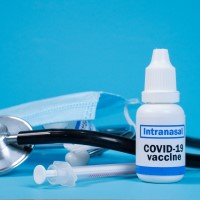
On Oct. 4, 2022, Washington University in St. Louis announced it had licensed the rights to develop, manufacture…

On Oct. 4, 2022, the United States Department of Agriculture’s (USDA) Agricultural Research Service (ARS), Southeast Poultry Research…
On Sept. 20, 2022, The health authorities in Uganda declared an outbreak of Ebola after a case of…

On Sept. 16, 2022, Novavax announced that the Taiwan Food and Drug Administration had granted expanded emergency use…

On Sept. 16, 2022, Novavax announced that the Israel Ministry of Health had granted an import and use…
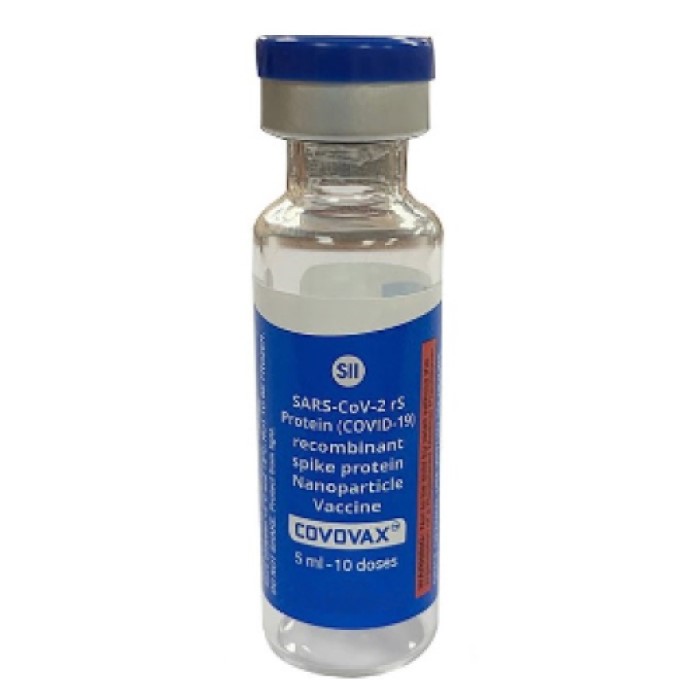
On Sept. 13, 2022, Novavax and Serum Institute of India, the world’s largest vaccine manufacturer by volume, announced…

On Sept. 7, 2022, Quest Diagnostics announced that it had received emergency use authorization (EUA) from the U.S….

On Sept. 2, 2022, Novavax announced that Swissmedic, the Swiss Agency for Therapeutic Products, had expanded its temporary…

On Aug. 23, 2022, Health authorities in the Democratic Republic of the Congo declared a resurgence of Ebola…
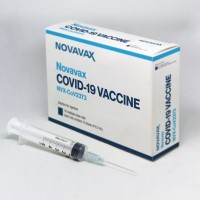
On Aug. 19, 2022, Novavax announced that the Novavax COVID-19 Vaccine, Adjuvanted (NVX-CoV2373) had received expanded emergency use…
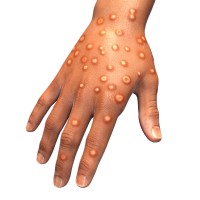
On Aug. 12, 2022, a group of global experts convened by WHO agreed on new names for monkeypox…

On Aug. 12, 2022, Novavax announced that partner, SK bioscience, had received a Post Approval Change Application approval…

On Aug. 9, 2022, the the Department of Health and Human Services (HHS) announced it had determined that…
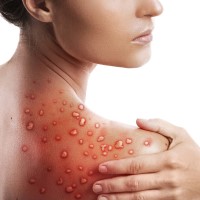
On Aug. 4, 2022, the U.S. Department of Health and Human Services declared the ongoing spread of monkeypox…
On Jul. 27, 2022, City of Hope announced that a 66-year-old man who was diagnosed with HIV in…

On Jul. 27, 2022, BD (Becton, Dickinson) announced their newly developed molecular polymerase chain reaction (PCR) test for…

On Jul. 26, 2022, Novavax announced that the Australian Therapeutic Goods Agency had granted expanded approval for provisional…

On Jul. 26, 2022, Novavax announced that Nuvaxovid (NVX-CoV2373) COVID-19 vaccine had received expanded manufacturing and marketing approval…
On Jul. 22, 2022, the Centers for Disease Control and Prevention (CDC) released a MMWR report that summarized…

On Jul. 17, 2022, Ghana announced the preliminary finding of two cases of Marburg virus disease and if…

On Jul 14. 2022, Aegis Sciences announced it is was authorized to begin testing for monkeypox using the…
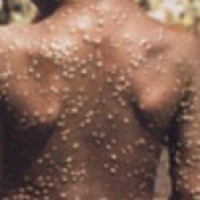
On Jul. 13, 2022, Quest Diagnostics announced that it had announced the nationwide availability* of the company’s lab-developed…