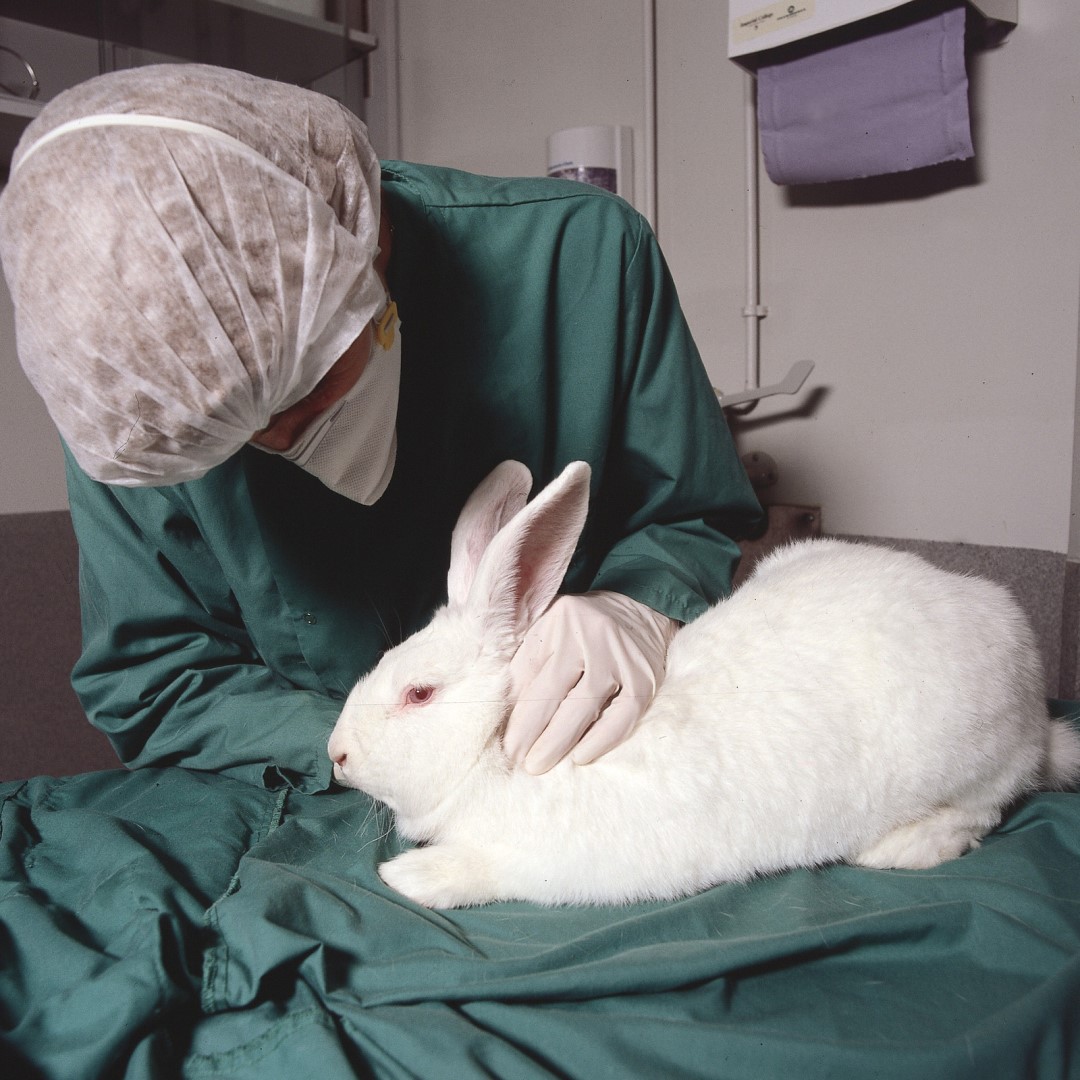
FDA Announces Plan to Phase Out Animal Testing Requirement for Monoclonal Antibodies and Other Drugs
On Apr. 10, 2025, the U.S. Food and Drug Administration announced it is taking a groundbreaking step to advance public health by replacing animal testing in the development of monoclonal antibody therapies and other drugs with more effective, human-relevant methods.
The new approach is designed to improve drug safety and accelerate the evaluation process, while reducing animal experimentation, lowering research and development (R&D) costs, and ultimately, drug prices.
The FDA’s animal testing requirement will be reduced, refined, or potentially replaced using a range of approaches, including AI-based computational models of toxicity and cell lines and organoid toxicity testing in a laboratory setting (so-called New Approach Methodologies or NAMs data).
Implementation of the regimen will begin immediately for investigational new drug (IND) applications, where inclusion of NAMs data is encouraged, and is outlined in a roadmap also being released today. To make determinations of efficacy, the agency will also begin use pre-existing, real-world safety data from other countries, with comparable regulatory standards, where the drug has already been studied in humans.
Working in close partnership with federal agencies such as the National Institutes of Health, the National Toxicology Program and the Department of Veterans Affairs, the FDA aims to accelerate the validation and adoption of these innovative methods through the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM).
Tags:
Source: U.S. Food and Drug Administration
Credit:
